Resources for PH Management
Explore the resources and tools for PH management in the Resource Center below.
By selecting “Add to my Request Form,” you can add materials to your Resource Center Request Form and they will be delivered to your practice, as well as get in contact with a United Therapeutics representative.
After you have made your selection, click the REQUEST NOW button below, which will take you to the form with your materials you have chosen.
PH management resources

PAH HCP Tool: REVEAL/ERS Risk Stratification and Calculator
Resource for HCPs with REVEAL/ERS risk stratification education and copies of calculator templates.
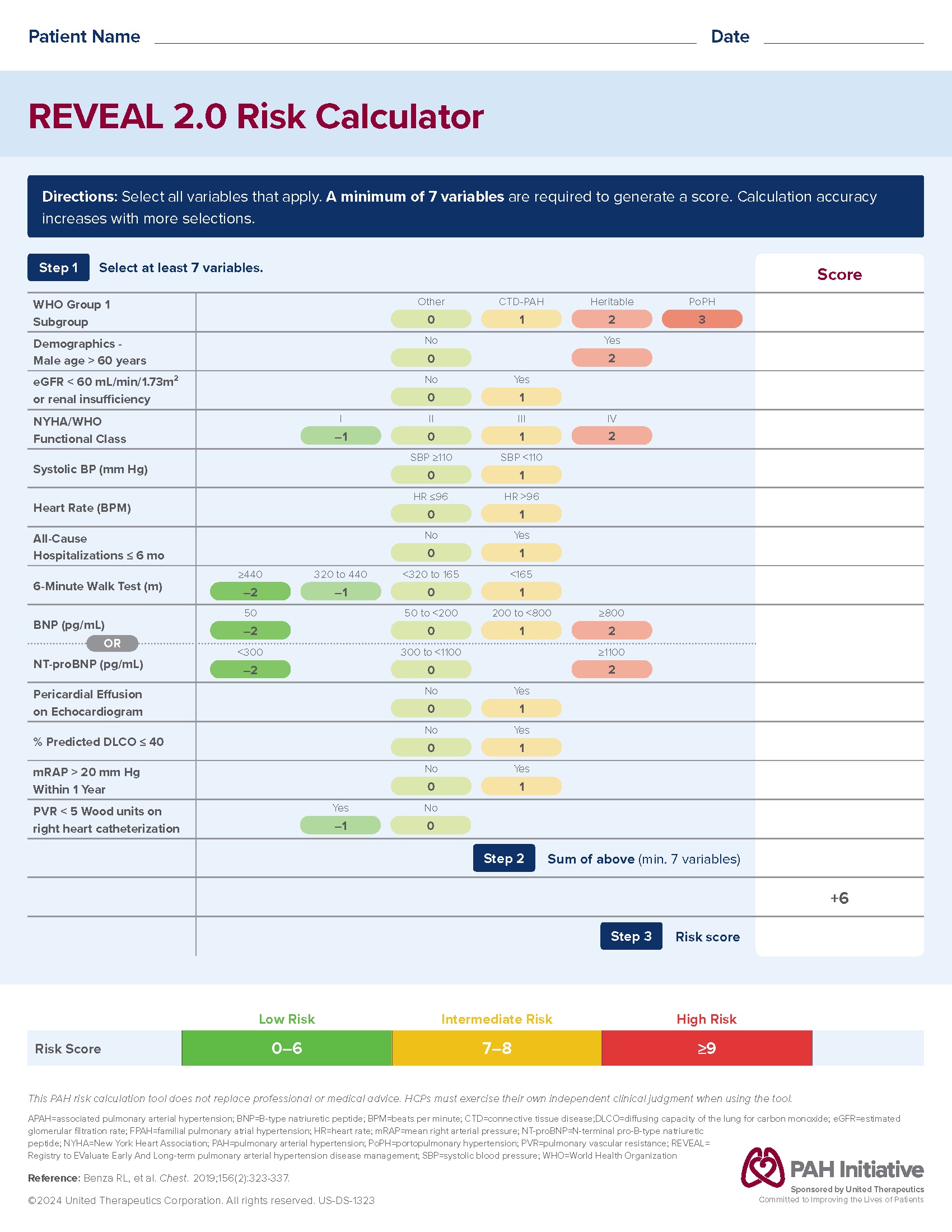
PAH HCP Tool: REVEAL/ERS Calculator
Resource for HCPs with copies of REVEAL/ERS calculator templates to help assess risk in patients with PAH.

PAH HCP Tool: Noninvasive/French Risk Stratification and Calculator
Resource for HCPs with risk stratification using French registry data and copies of calculator templates.

PAH Today Magazine for Patients
The latest issue of PAH Today magazine, full of insights and inspiration for PAH patients and their caregivers.

PAH Patient Video: PAH Basics
Dr. Lana Melendres-Groves, a PAH expert, explains PAH and provides details on the cardiovascular system.

PAH Patient Video: PAH Treatment Goals
Dr. Lana Melendres-Groves describes treatment goals and how they can help assess if treatment is working well.

PAH Patient Video: PAH Treatment Options
Education on risk status, PAH medication classes, and treatment options with Dr. Lana Melendres-Groves.
PAH Patient Video: Understanding PAH
Explanation of how PAH affects the heart and lungs, how it progresses, and how treatments work within 3 key pathways.

PAH Patient Waiting Room Checklist
Questions and details patients with PAH should consider while preparing to visit their HCP.

HCP 6-Minute Walk Test “How-to” Guide
Standardize how your practice measures patients’ 6MWD.
HCP Echocardiogram and Right Heart Tear Pad
Key right heart parameters relevant to PAH management via echo.

Patient PAH Disease Education Brochure
Unbranded PAH educational brochure covers PAH basics, considerations for care, and treatment pathways. A flash drive with educational videos is included.

Right Heart Catheterization Patient Brochure – English
Brochure to help educate your patients about the right heart catheterization procedure.

Right Heart Catheterization Patient Brochure – Chinese
Right heart catheterization brochure translated for your native-Chinese (Mandarin) speaking patients.

Right Heart Catheterization Patient Brochure – Portuguese
Right heart catheterization brochure translated for your native-Portuguese (Brazilian) speaking patients.

Right Heart Catheterization Patient Brochure – Vietnamese
Right heart catheterization brochure translated for your native-Vietnamese speaking patients.

Right Heart Catheterization Patient Brochure – French
Right heart catheterization brochure translated for your native-French (French Canadian) speaking patients.
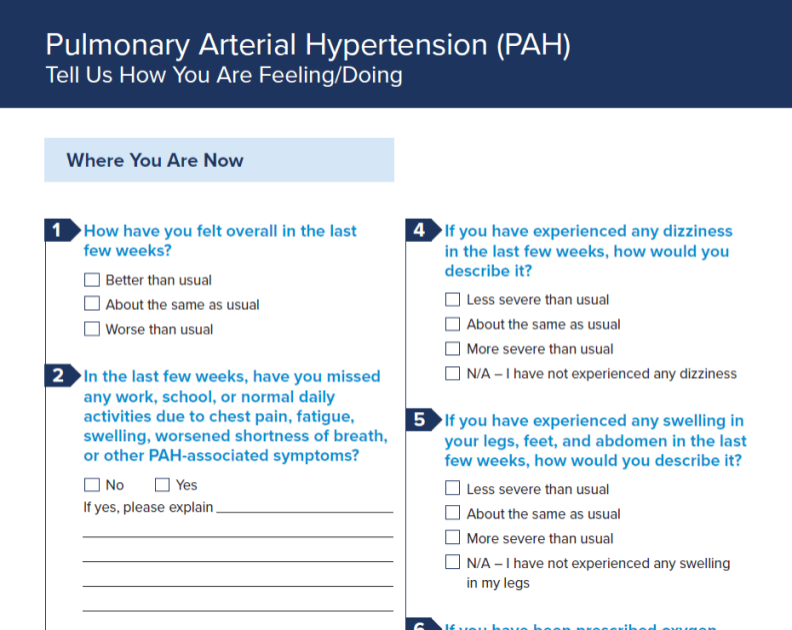
Patient Intake Form – English
Use this form to better understand how your patients are feeling.

Patient Intake Form – Chinese
Patient intake form translated for your native-Chinese (Mandarin) speaking patients.
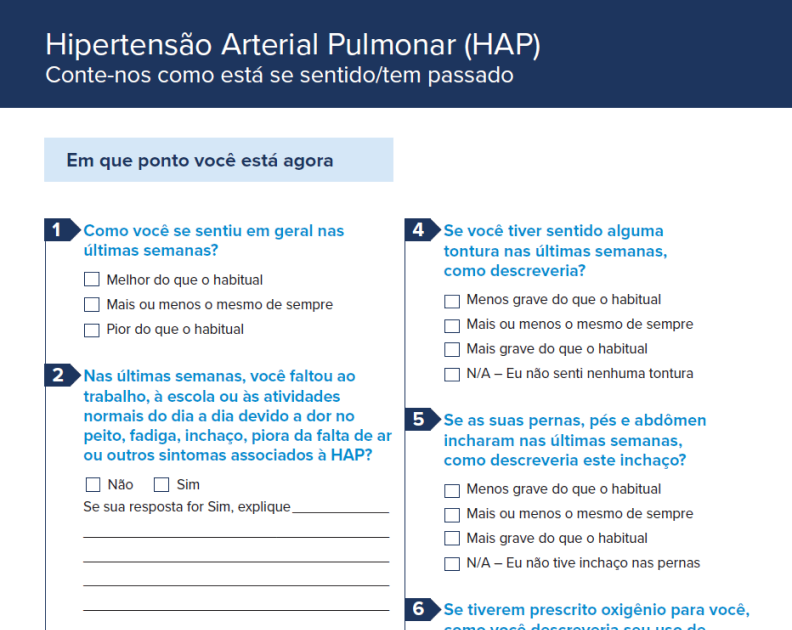
Patient Intake Form – Portuguese
Patient intake form translated for your native-Portuguese (Brazilian) speaking patients.

Patient Intake Form – Vietnamese
Patient intake form translated for your native-Vietnamese speaking patients.
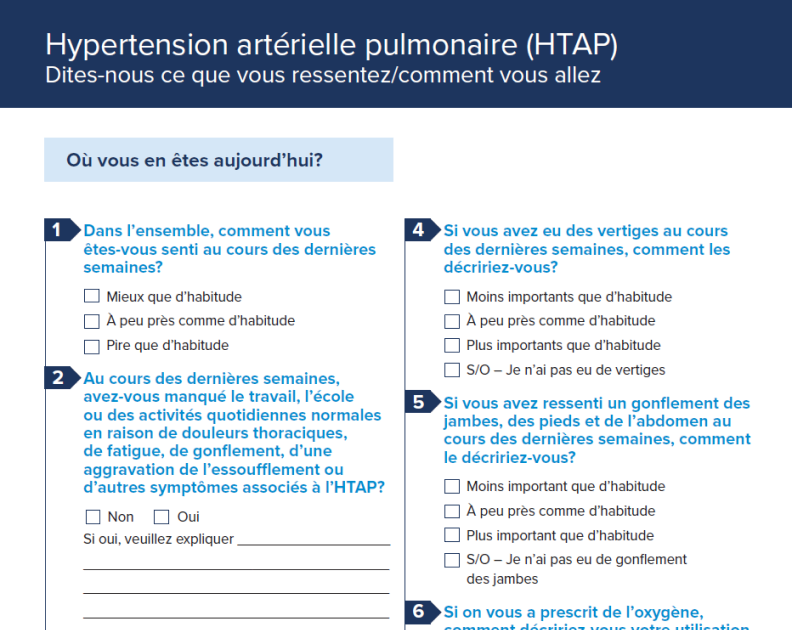
Patient Intake Form – French
Patient intake form translated for your native-French (French Canadian) speaking patients.
Request a Rep
To speak with a United Therapeutics sales representative, complete and submit this form.
6MWD=6-minute walk distance; ERS=European Respiratory Society; PAH=pulmonary arterial hypertension; PH=pulmonary hypertension; REVEAL=Registry to Evaluate Early and Long-Term PAH Disease Management.