Resources for PH Therapies
Explore the clinical and patient materials for our PH therapies—Orenitram, TYVASO, and Remodulin—in the Resource Center below.
By selecting “Add to my Request Form,” you can add materials to your Resource Center Request Form and they will be delivered to your practice, as well as get in contact with a United Therapeutics representative.
After you have made your selection, click the REQUEST NOW button below, which will take you to the form with your materials you have chosen.

Orenitram® resources

Orenitram Patient Brochure: Considering Orenitram
A guide for patients considering Orenitram to learn why treatment may be right for them and more.

Orenitram Patient Brochure: What to Expect
A guide for patients to learn what to expect between the time they are prescribed Orenitram and when they receive their first shipment of drug.

Orenitram HCP Brochure:
Getting StartedAn informative brochure highlighting 3 key steps to successfully get patients started on Orenitram.

Orenitram HCP and Patient Package: Side Effect Management
Brochures for healthcare providers and patients to learn more about Orenitram side effects and the best strategies to manage them.

Orenitram HCP and Patient Package: 90-Day Trial Program
An informational package for patients who want to try Orenitram at no cost for up to 90 days.

Orenitram HCP Referral Form
A referral form and 3-step process to authorize patients to receive Orenitram from their Specialty Pharmacy.
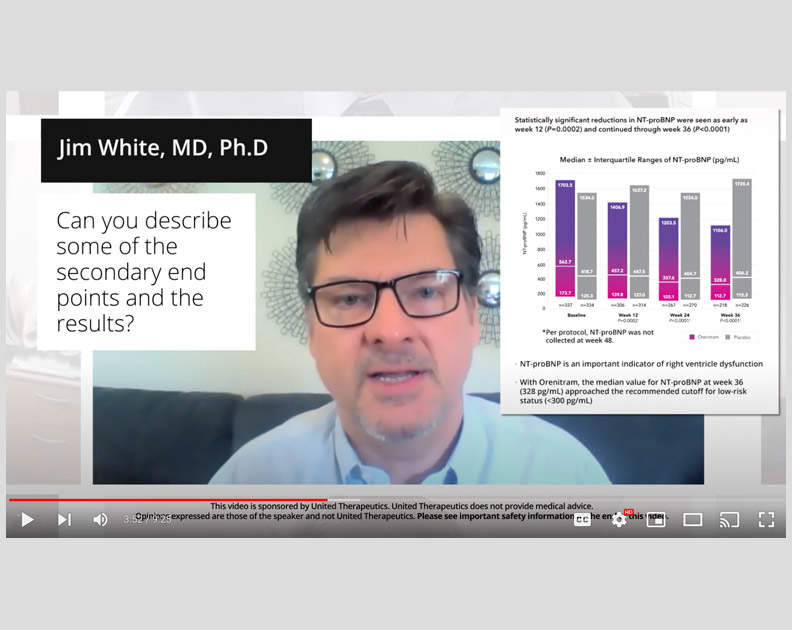
Orenitram HCP Video: Overview of FREEDOM-EV
The principal investigator of FREEDOM-EV, R. James White, MD, PhD, describes the trial, key patient characteristics, and important outcomes.
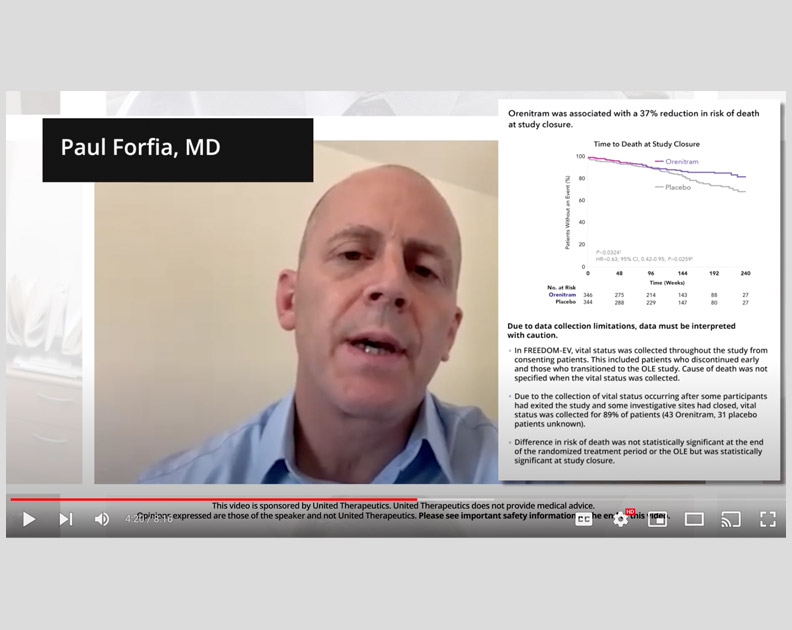
Orenitram HCP Video: Right Heart Function in FREEDOM-EV
Paul Forfia, MD, on the relationship between right heart dysfunction and clinical worsening, and the impact of Orenitram in the FREEDOM-EV trial.
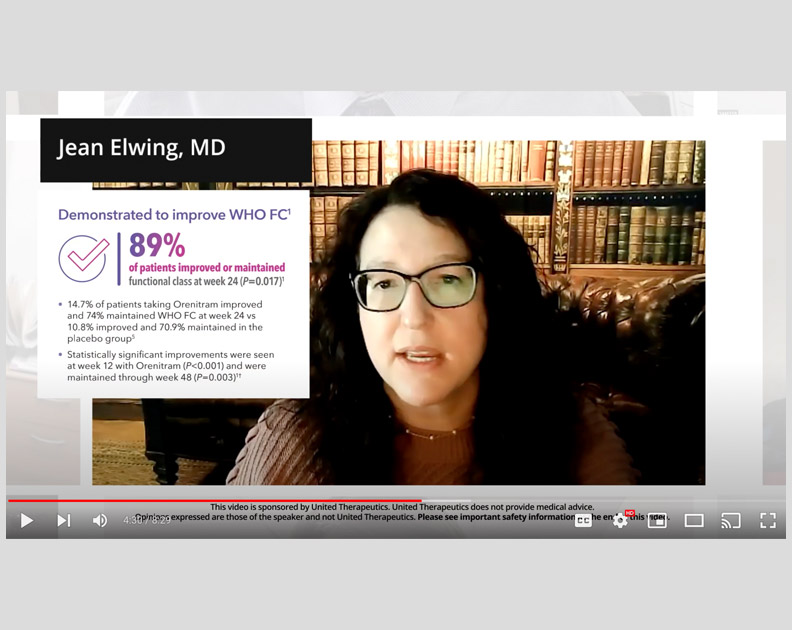
Orenitram HCP Video: Measuring Risk and the Impact of Orenitram
Jean Elwing, MD, on the effect that Orenitram had on several key parameters of risk, as well as overall risk status in the FREEDOM-EV trial.
Orenitram HCP Video: Dosing and AE Management as a Team
Franck Rahaghi, MD, and Starlet Harrimon, RN, on working with patients, lessons from FREEDOM-EV, and using risk assessments with Orenitram.
Orenitram Patient Video:
Luisa’s ExperienceLearn how Luisa works with her healthcare team to balance titration and side effects.
Orenitram Patient Video:
Jenny’s ExperienceWatch Jenny discuss how she and her doctor developed a dosing plan that aligns with her treatment goals.
-Translation-1.jpg)
Patients Considering Orenitram Canadian French Translation
A guide for patients considering Orenitram to learn why treatment may be right for them and more. Translated in Canadian French.

Patients Considering Orenitram Vietnamese Translation
A guide for patients considering Orenitram to learn why treatment may be right for them and more. Translated in Vietnamese.
-Translation-1.jpg)
Patients Considering Orenitram Chinese (China) Translation
A guide for patients considering Orenitram to learn why treatment may be right for them and more. Translated in Chinese.

Patients Considering Orenitram Brazilian Portuguese Translation
A guide for patients considering Orenitram to learn why treatment may be right for them and more. Translated in Brazilian Portuguese.
TYVASO® for PAH resources

TYVASO PAH Patient Brochure
Informational booklet for patients that provides details on PAH, treatment, administration tips, and more.

TYVASO Patient Video: How to Use the TYVASO Nebulizer
Learn more about how to use the TYVASO nebulizer.

TYVASO Patient Instructions for Use
TYVASO Instructions for Use manual with details on programming, safety, cleaning, and storage.
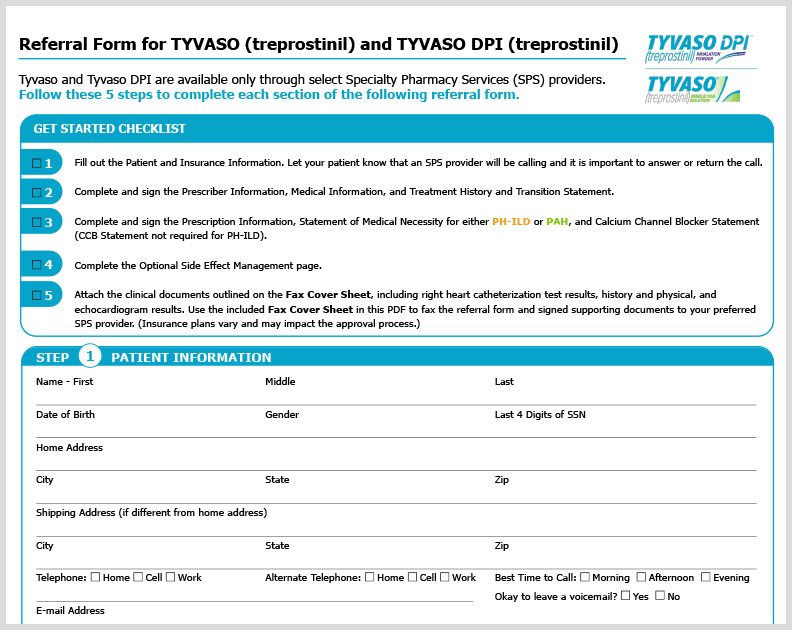
TYVASO HCP Referral Form
A referral form for HCPs outlining a 5-step process to ensure patients receive TYVASO treatment, including TYVASO DPI.
TYVASO Patient Video: How to Use TYVASO DPI
Learn more about how to use TYVASO DPI for the treatment of PAH.

TYVASO HCP Video: About TYVASO DPI
See an overview of TYVASO DPI, including the benefits and how it works.

TYVASO DPI Patient Instructions for Use
TYVASO DPI Instructions for Use manual with details on how to use TYVASO DPI.

TYVASO DPI Patient Quick Tips Guide
Read tips on how to use TYVASO DPI.

TYVASO DPI Patient Titration Guide
Learn more about titrating to target dose with TYVASO DPI.

TYVASO Nebulizer Patient Quick Tips Guide
Read tips on how to use the TYVASO nebulizer.
-translation-1.jpg)
TYVASO PAH Patient Brochure Canadian French Translation
Informational booklet for patients that provides details on PAH treatment, administration tips, and more. Translated in Canadian French.

TYVASO PAH Patient Brochure Vietnamese Translation
Informational booklet for patients that provides details on PAH treatment, administration tips, and more. Translated in Vietnamese.
-Translation-1.jpg)
TYVASO PAH Patient Brochure Chinese (China) Translation
Informational booklet for patients that provides details on PAH treatment, administration tips, and more. Translated in Chinese.

TYVASO PAH Patient Brochure Brazilian Portuguese Translation
Informational booklet for patients that provides details on PAH treatment, administration tips, and more. Translated in Brazilian Portuguese.
TYVASO® for PH-ILD resources

TYVASO PH-ILD Patient Brochure
Informational booklet for patients that provides details on PH-ILD treatment, administration tips, and more.
TYVASO PH-ILD Patient Video: Catherine’s Story
Hear from Catherine, a real TYVASO patient, about her experiences with TYVASO and the impact it has had on her.
TYVASO PH-ILD Patient Video: Tina’s Story
Hear from Tina and her experience with titrating with TYVASO.
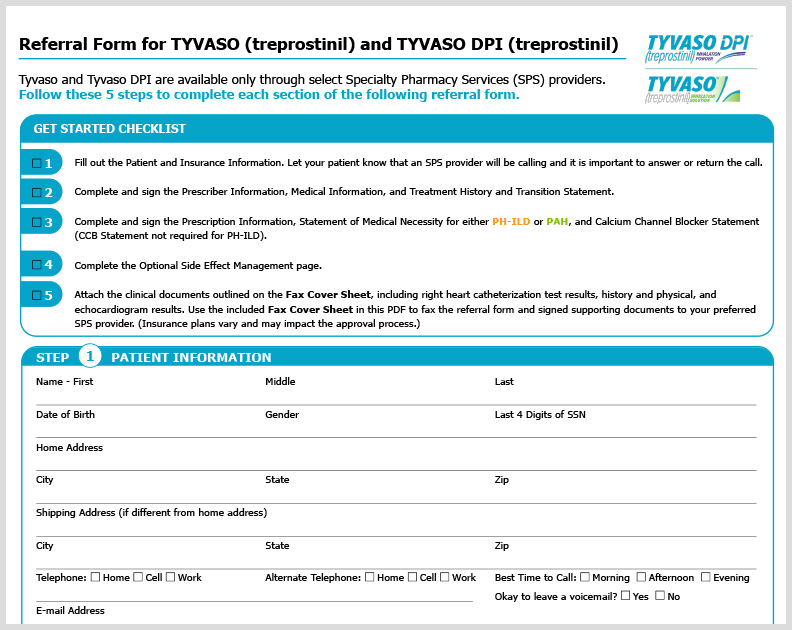
TYVASO PH-ILD HCP Referral Form
A referral form for HCPs outlining a 5-step process to ensure patients receive TYVASO treatment, including TYVASO DPI.

TYVASO DPI Patient Instructions for Use
TYVASO DPI Instructions for Use manual with details on how to use TYVASO DPI.

TYVASO Patient Instructions for Use
TYVASO Instructions for Use manual with details on programming, safety, cleaning, and storage.
-translation-1.jpg)
TYVASO PH-ILD Patient Brochure Canadian French Translation
Informational booklet for patients that provides details on PH-ILD treatment, administration tips, and more. Translated in Canadian French.

TYVASO PH-ILD Patient Brochure Vietnamese Translation
Informational booklet for patients that provides details on PH-ILD treatment, administration tips, and more. Translated in Vietnamese.
-Translation-1.jpg)
TYVASO PH-ILD Patient Brochure Chinese (China) Translation
Informational booklet for patients that provides details on PH-ILD treatment, administration tips, and more. Translated in Chinese.

TYVASO PH-ILD Patient Brochure Brazilian Portuguese Translation
Informational booklet for patients that provides details on PH-ILD treatment, administration tips, and more. Translated in Brazilian Portuguese.
Remodulin® resources
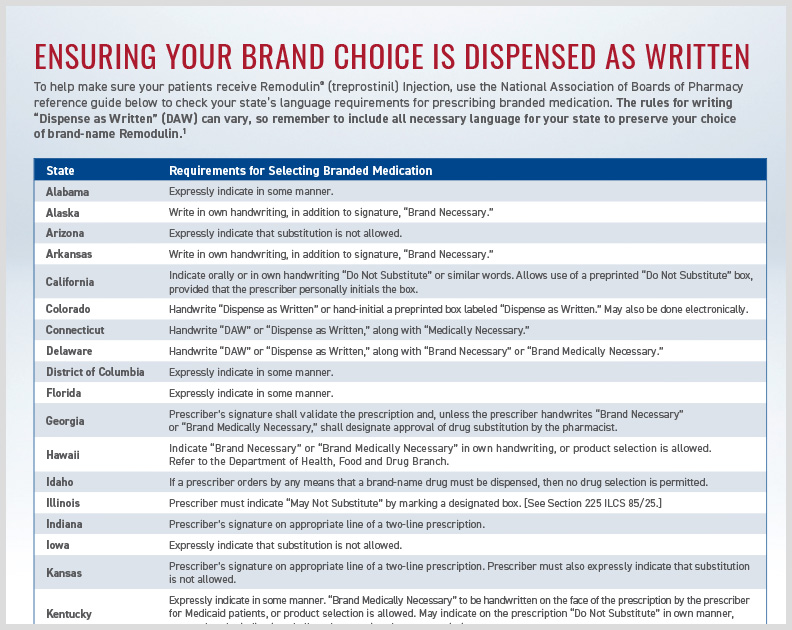
Remodulin HCP DAW State Language
Outlines state-specific requirements for “Dispense as Written” (DAW) to prescribe brand name Remodulin.
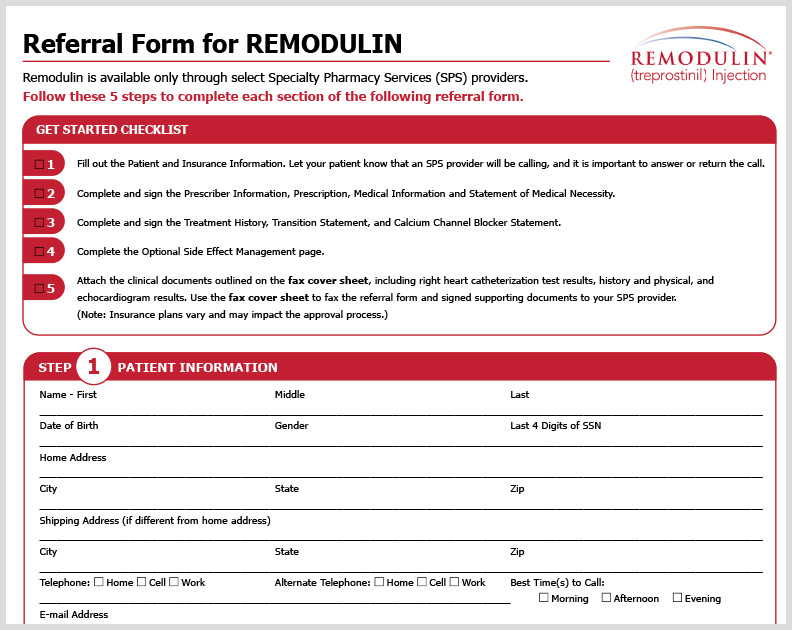
Remodulin HCP Referral Form
A referral form to fill out for patients to receive Remodulin through Specialty Pharmacies.
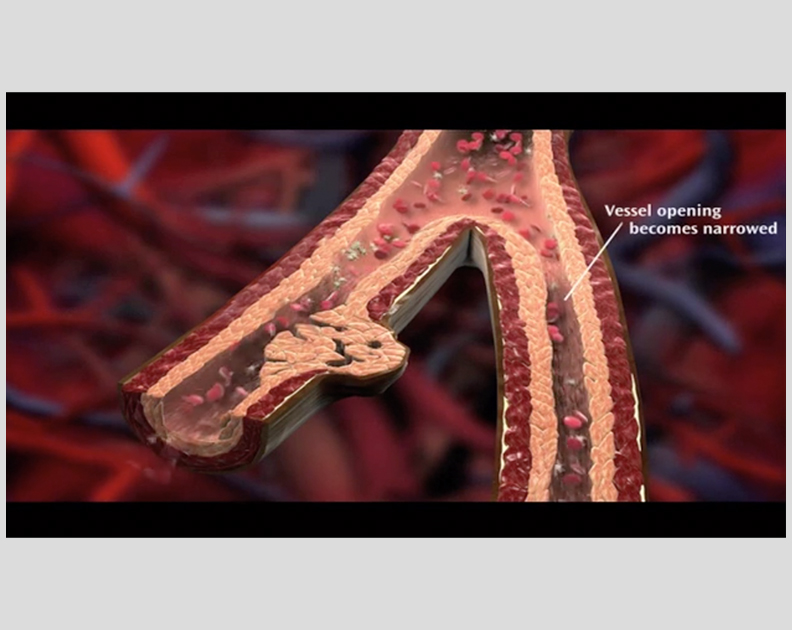
Remodulin Patient Video: How Blood Flows in the Vessels in the Lungs
See what happens to the flow of blood in your lungs with PAH.
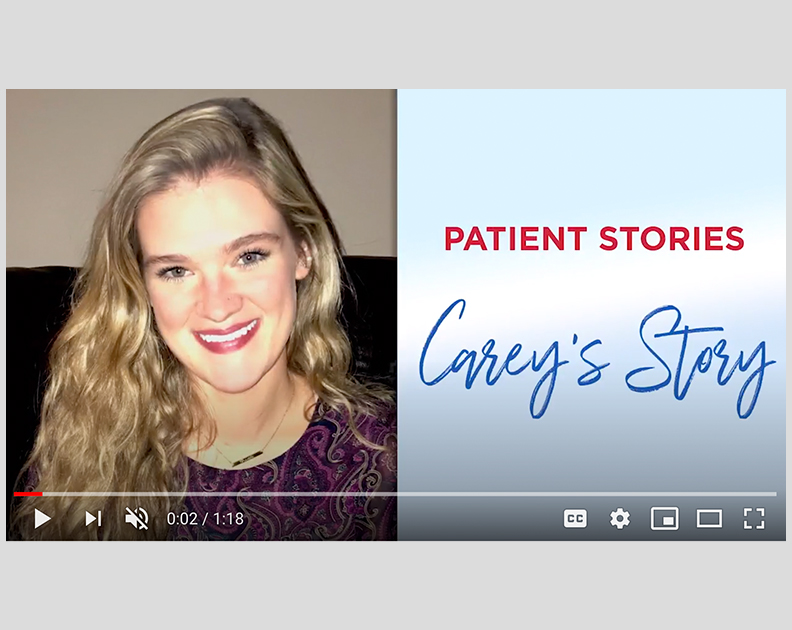
Remodulin Patient Video: Carey’s Story
PAH patient Carey shares her story about how she persevered and didn’t let her disease define her.

Remodulin Patient Video: Helpful Information About Remodulin
Dr. Sean Studer and nurse Glenna Traiger review Remodulin benefits, risks, administration, and side effects.

Remodulin Patient Video: PAH Life Hacks—Showering With Your Pump
PAH patient Carey shares her tips on how to protect a pump in the shower.

Remodulin Patient Video: PAH Life Hacks—Traveling With Your Pump
PAH patient Wendy shares her advice that made traveling with a Remodulin pump more manageable for her.

Remunity HCP and Patient Video: Remunity® User Guide—First-Time Setup
A video on setting up the Remunity pump, including pairing pump to remote and setting a delivery rate.
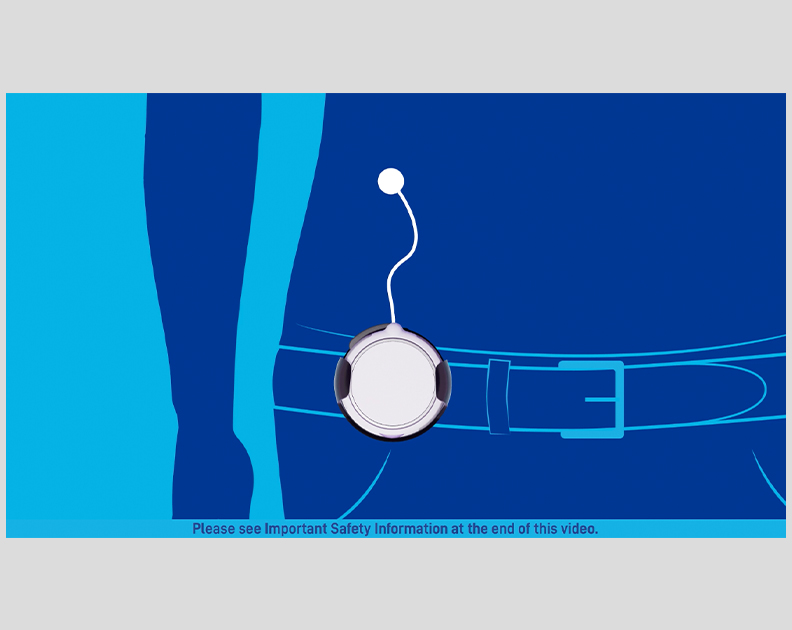
Remunity HCP and Patient Video: Remunity® User Guide—Connecting Your First Cassette
A video on how to prime a Remodulin cassette, connect it to the pump, and connect the pump to the catheter.

Remunity HCP and Patient Video: Remunity® User Guide—Cassette Change
A video on how to disconnect infusion sets before changing a cassette, replace the pump battery, and reconnect devices.

Remunity HCP and Patient Video: Remunity® User Guide—Pump Alerts and Alarms
A video showing what Remunity pump notifications, alerts, and alarms mean and what to do when they occur.
Remunity HCP and Patient Video: Remunity® User Guide—Troubleshooting and FAQs
A video about how to troubleshoot if the pump isn’t functioning properly, including answers to frequently asked questions.
Remodulin HCP and Patient Video: Remodulin SC Site Pain
Patients and HCPs talk about injection site pain with Remodulin SC and ways to help manage it.
-Translation-1.jpg)
Remodulin Patient Brochure Canadian French Translation
This brochure helps patients understand treatment with Remodulin and the resources available to them through United Therapeutics. Translated in Canadian French.

Remodulin Patient Brochure Vietnamese Translation
This brochure helps patients understand treatment with Remodulin and the resources available to them through United Therapeutics. Translated in Vietnamese.
-Translation-1.jpg)
Remodulin Patient Brochure Chinese (China) Translation
This brochure helps patients understand treatment with Remodulin and the resources available to them through United Therapeutics. Translated in Chinese.

Remodulin Patient Brochure Brazilian Portuguese Translation
This brochure helps patients understand treatment with Remodulin and the resources available to them through United Therapeutics. Translated in Brazilian Portuguese.
Request a Rep
To speak with a United Therapeutics sales representative, complete and submit this form.
PH management resources
To access and download clinical materials for general PH management, visit the PH Management Resource Center.
AE=adverse event; DPI=dry powder inhaler; MOA=mechanism of action; PAH=pulmonary arterial hypertension; PH=pulmonary hypertension; PH-ILD=pulmonary hypertension associated with interstitial lung disease; SC=subcutaneous.